Vanilloid versus isovanilloid: IPB scientists develop tailored enzymes with modified regiospecificity.
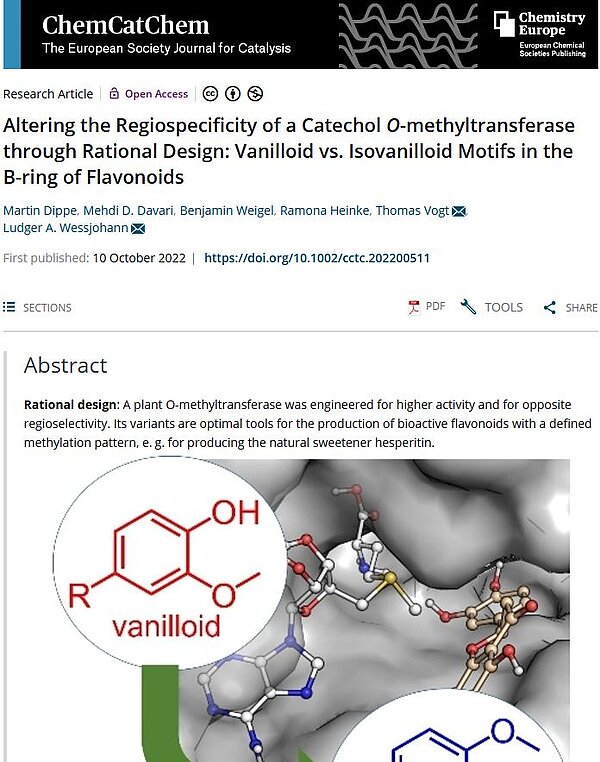
Although the biocatalytic production of plant and synthetic bioactives in reaction tubes is gaining increasing importance, it still holds a lot of potential for development and refinement. The targeted production of desired compounds or substance groups is currently limited by the fact that many enzymes with particular substrate and regiospecificities have not yet been discovered, isolated, made available, or do not even exist. One solution to this problem is to adapt existing enzymes already in use to the requirements of the planned biocatalysis. Such a rational re-design has recently been achieved by IPB scientists for a catechol-O-methyltransferase from the ice plant (Mesembryanthemum crystallinum). The enzyme catalyzes the methylation of various flavonoid compounds, whereby the methyl group is attached exclusively to the hydroxyl of the 3rd C atom in the catechol group, i.e. at the meta position. As a result of this strict 3'-selective regiospecificity, a vanilloid motif is formed on the flavone molecule. The Halle scientists exchanged several amino acids in the substrate pocket of the enzyme by site-directed mutagenesis and thus obtained new enzyme variants with opposite, i.e., 4′-para-regioselectivity or with improved catalytic efficiency. Accordingly, the 4'-selective methyltransferases produced an isovanilloid motif at the basic stem of the flavonoid compounds.
This change in regiospecificity and thus in the methylation pattern at the substrate leads of course to different end products, as the Halle scientists showed in the specific example of eriodictyol. Thus, the methylation of eriodictyol at the 3'-hydroxyl results in the compound homoeriodictyol, a bitter-masking flavone known from Eriodictyon californicum, the American sacred herb. On the other hand, when eriodictyol is methylated at the 4'-hydroxyl it forms hesperetin, a natural sweetener found in orange peels. As a potent antioxidant, hesperetin inhibits lipid peroxidation and has neuroprotective effects. The new enzyme variants have also been successfully tested on other flavonoid substrates.
In general, chemo- and regioselective O-methylation of catechol units in complex molecules is considered an important goal in the synthesis of bioactive compounds. Production of the methylated natural products in an enzymatic way, without protection and deprotection strategies and without toxic solvents, but only in water, is one possibility of green chemistry to achieve this goal. And tailor-made enzymes as optimal tools for biocatalytic production of natural and active compounds with defined methylation patterns will be essential for this purpose. This is because the future biocatalytic syntheses of flavonoids, indoles, benzylisoquinolines and methyl esters as starting products for fragrances and drugs depend on the availability of methyltransferases with suitable substrate selectivities as well as product and regiospecificities.